Caught in the Flow: When the Right Adrenal Gland Takes an ad-vena-ture
Signalment
- Age: 11 years old
- Gender: Male
- Species: Canine
- Breed: Rottweiler
- Weight: 40 kg
History
The patient presented to the emergency hospital for having difficulty walking, which progressed to complete unwillingness to stand up after being let outside. The owner reported that he eventually was able to stand but struggled to get up and would continue to fall over or refuse to move. He received his first Librela injection 2 weeks prior. The owner reported a significantly decreased appetite with normal water intake. He takes Simparica Trio and is up to date on core vaccines.
The owner reports that the patient was diagnosed with a liver issue 4 years ago but has since recovered. No medication or supplements are taken. The normal diet is Royal Canin Urinary S/O.
Ultrasound Findings
| Kidneys | Normal size (Lt/Rt = 9.0/8.9 cm) and shape with normal corticomedullary dimensions. There is punctate mineralization throughout the renal cortices. No pyelectasia visualized. Kidney length: Lt/Rt: 9.0 / 8.9 cm Aorta diameter at the level of the Lt/Rt Kidney: 1.2 / 1.2 cm. Kidney length: Aortic diameter Lt/Rt: 7.5 / 7.4 (Normal 5.5-9.1) |
| Spleen | Normal size (1.8 cm in depth), shape, and echogenicity. There are occasional, non-capsule deforming, hypoechoic nodules throughout the spleen measuring up to 0.5 (D) x 0.7 (L) cm. |
| Urinary Bladder | The bladder is moderately distended with anechoic urine and is of relatively normal contour and thickness. There is a mild amount of hyperechoic sediment on the gravity-dependent portion of the bladder wall. No overt obstruction, uroliths, or neoplasia noted. |
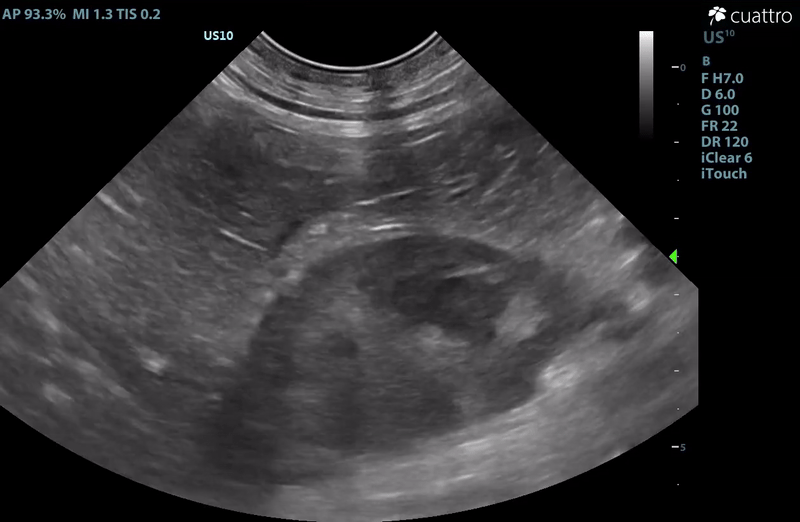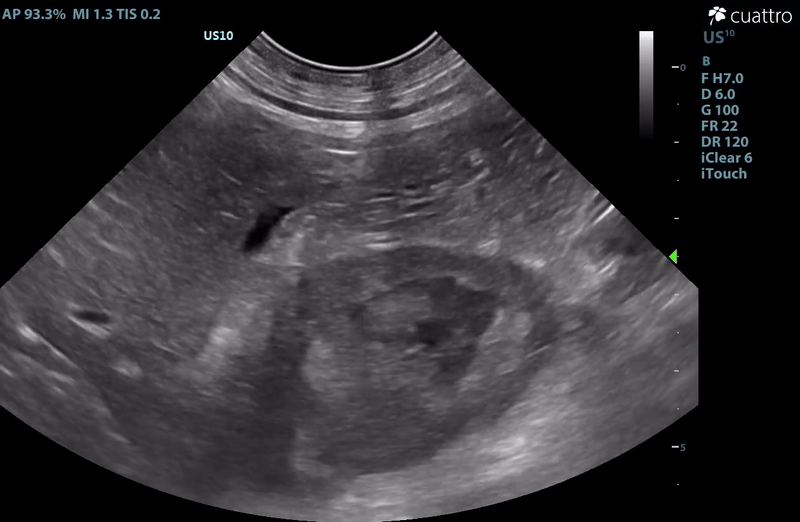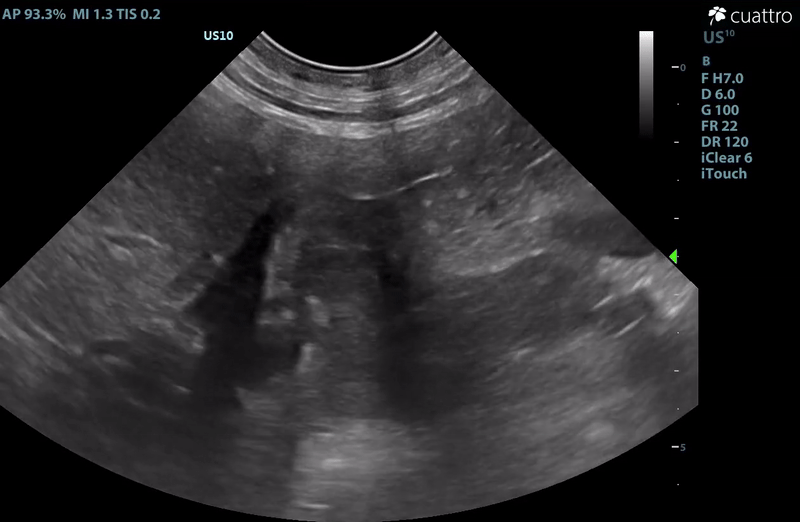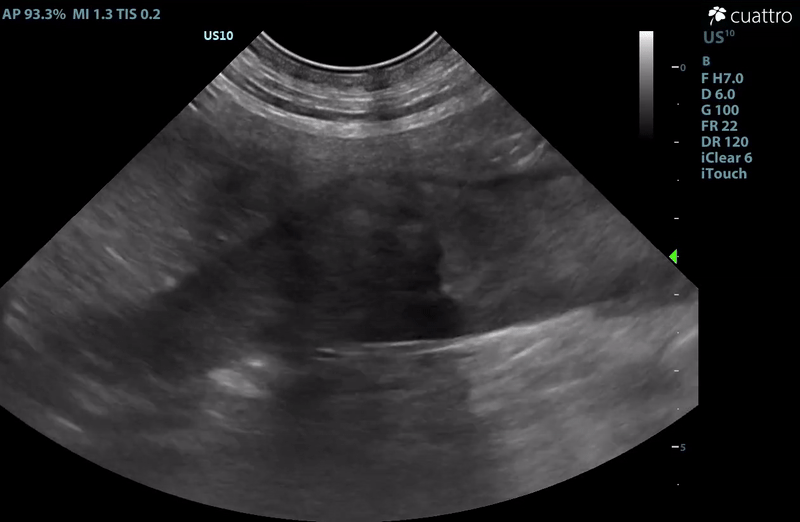
| Adrenal Glands | The left adrenal gland was visualized and recognized as having a mildly rounded "plump" shape, with increased size (Ltcd = 8.5 mm), and normal position and echogenicity for this breed. No adrenal invasion into the vena cava, phrenic vein thrombosis, or clinically significant nodular changes were noted. There is punctate mineralization throughout the adrenal gland. The right adrenal gland was visualized as having an abnormally irregular, mass-like appearance, with mixed echogenicity and areas of mineralization and cavitation [1.2 (D) x 1.9 (L) cm]. There is marked enlargement, measuring at least 2.8 (D) x 5.1 (L) cm. Significant invasion from the dorsal aspect of the cranial pole extending into the caudal vena cava (CVC) is visualized. Within the CVC lumen, the right adrenal mass measures at least 2.3 (D) x 5.1 (L) cm, occupying ~95-98% of the lumen (subjectively). In certain views, the mass appears to distort the caval walls outwardly. Caudal to the intraluminal mass, 'smoke' (spontaneous echo contrast) is noted. Assessment with color and spectral doppler showed evidence of an obstructive flow pattern with minimal forward flow evident along the ventral aspect of the cava and a non-pulsatile, bi-directional flow profile through the remaining majority of the lumen when measured directly caudal to the mass. Cranial to the mass, there is pulsatile caval flow with elevated flow velocity (1.3 m/s), exceeding aortic flow velocity (0.8 m/s). |
| Normal adrenal gland size by weight: |
|
| Intestinal Tract | The stomach is empty and collapsed with normal rugal folds and layering. The pylorus is free of obstruction. The duodenum is mildly thickened (5.6 mm) with normal bowel layering and decreased motility. The remaining small intestinal loops have normal bowel layering, thickness, and motility. No abnormal layering, obstruction, or masses seen. The colon has high normal wall thickness and layering throughout. Maximum thickness measurements: Duodenum - 5.6 mm; Jejunum - 4.0 mm; Ileum - 3.4 mm; Colon - 2.4 mm |
| Lymph Nodes | The medial and internal iliac lymph nodes are mild to moderately enlarged with mildly rounded shape having homogenous hypoechoic echogenicity and punctate mineralization throughout. LMILN: 1.2 (D) x 4.0 (L) cm RMILN: 1.4 (D) x 3.0 (L) cm IILN: 0.6 (D) x 2.5 (L) cm |
| Serosal Surfaces | Scant anechoic ascites was noted around the right adrenal gland. No focal lesions or changes in mesenteric echogenicity noted. |
| Prostate | Moderate generalized enlargement (5.9 cm in depth) with hyperechoic coarse echogenicity and multifocal anechoic small cysts throughout. No mineralization or focal lesions. |
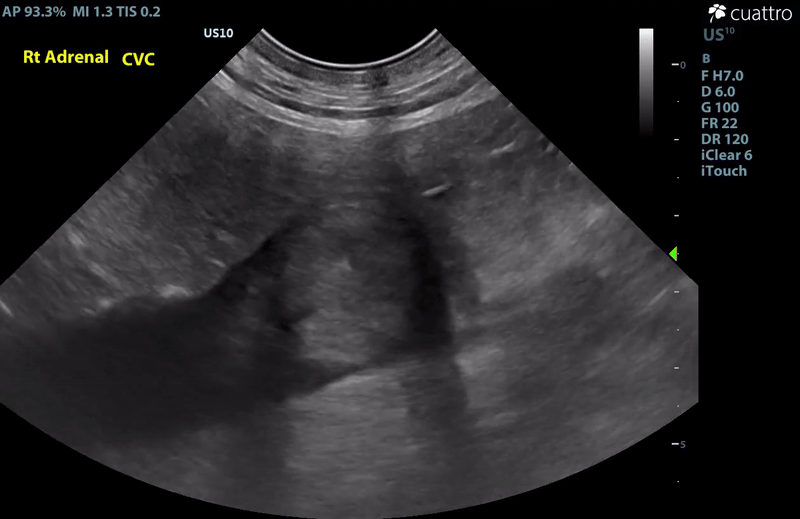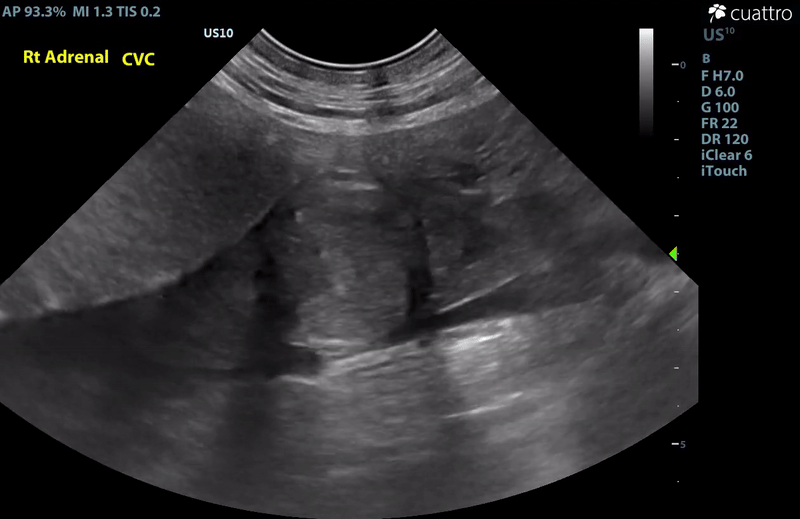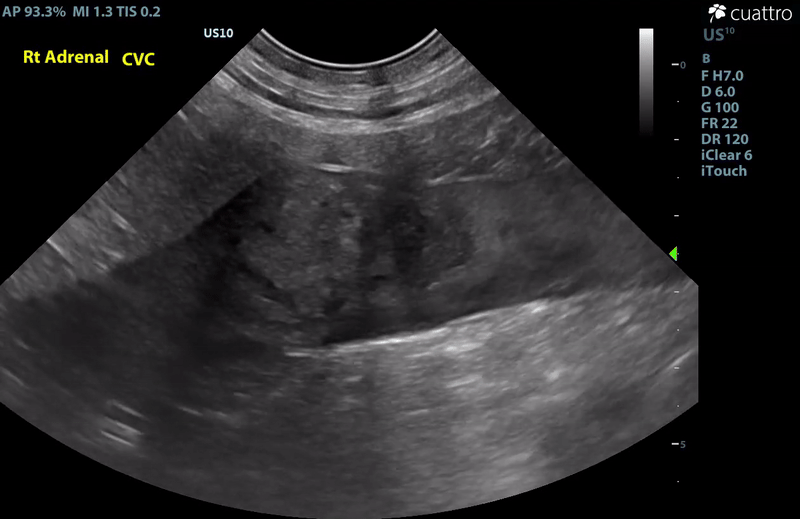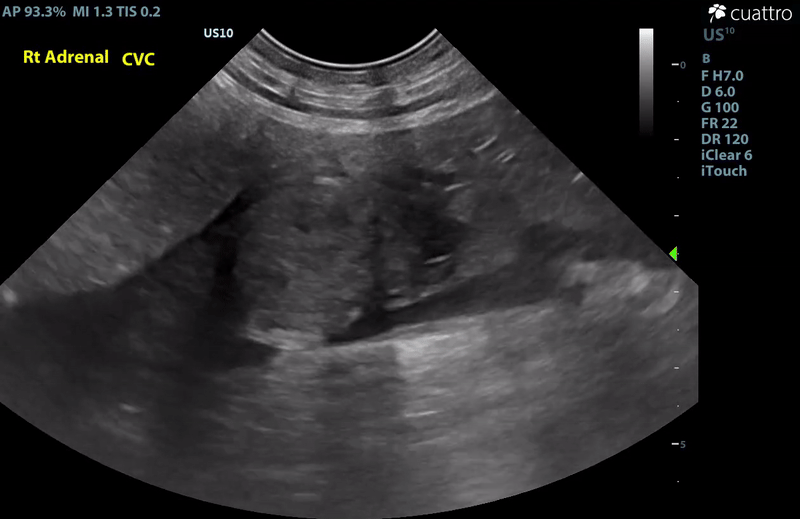
Abdominal Ultrasound Interpretation
- Right Adrenal Mass with Caudal Vena Cava invasion/partial obstruction DDx: differentials include cortical & medullary neoplasia vs. benign hyperplasia
-
- Cortical Neoplasia - Adrenal adenocarcinoma = Cushing's Dz (common)
- Non-functional adenoma vs. myelolipoma vs. hyperplasia
- Medullary Neoplasia - Pheochromocytoma (rare)
- Functional adenoma
- Other neoplasia
-
- Left Adrenomegaly DDx: - the findings are mild - DDx: enlarged adrenal glands are suggestive hyperadrenocorticism. If the patient doesn't have clinical signs for hyperadrenocorticism, stress may be the etiology for the adrenomegaly.
- Splenic Nodules - the findings are mild - Non-neoplastic lesion (Extramedullary hematopoiesis, Hematoma, nodular hyperplasia, granuloma, abscess) vs. Hemangioma vs. Lymphoma vs. Mast Cell Tumor vs. Splenic Sarcoma vs. Malignant Histiocytosis vs. Metastatic lesion
- Lymph nodes (medial and internal iliac) - the findings are mild to moderate - DDx: reactive vs. infection vs. IBD vs. infiltrative neoplasia (lymphoma vs. mast cell vs. other) vs. metastatic neoplasia
- Duodenal thickening - the findings are mild - DDX: reactive duodenitis vs. inflammatory bowel disease vs. food allergy/intolerance vs. infiltrative neoplasia (unlikely).
- Ileus (duodenal) - Functional (inflammatory disease vs. pancreatitis vs. peritonitis vs. other) vs. Metabolic (uremia vs. endotoxemia vs. other) vs. neuromuscular vs. Mechanical (Foreign body vs. adhesions vs. neoplasia vs. other)
- Prostate - the findings are mild to moderate - DDx: benign prostatic hyperplasia vs. prostatitis (bacterial vs. sterile) vs. prostatic neoplasia (adenocarcinoma vs. transitional cell carcinoma arising from the urethra and prostatic duct epithelium).
- Echogenic urine - the findings are mild - Ddx: cellular vs. lipid vs. protein vs. amorphous debris
- Ascites - this finding is scant - DDx: transudate vs. hemorrhagic vs. exudate
Recommendations
– The right adrenal changes are most consistent with, and highly concerning for neoplasia. Unfortunately, the marked enlargement and invasive behavior are strong indicators or malignancy. The most commonly reported invasive adrenal tumors are functional cortisol secreting tumors and pheochromocytomas. Given contralateral adrenomegaly and lack of ultrasonographic changes commonly associated with hyperadrenocorticism, pheochromocytoma would be more likely in this case. Consider urinary metanephrine testing to assess further. A LDDS test could also be considered to help rule out a cortisol secreting tumor (adenocarcinoma).
– Regardless of tumor type, referral of this patient to a veterinary surgeon to discuss possible therapeutic options is highly recommended. Advanced imaging (i.e. CT scan) is indicated to assess degree of cava wall invasion (dictating surgical feasibility) and to assess further for metastatic disease.
– The splenic nodules noted are small and non-capsule deforming. These changes are most consistent with extramedullary hematopoiesis or other benign changes. Neoplasia is not highly suspected, however, given the additional findings, there is an increased risk that these nodules are of a neoplastic etiology. Splenectomy could be considered if surgery is pursued, however, this may be superfluous given the ultrasonographic appearance.
– The significance of the medial and internal iliac lymphadenopathy is unclear and likely multifactorial. Given the reported UTI, this may be, in part, reactive in nature. Additionally, the degree of Rocko's caval obstruction is likely causing elevated pressures within the cava, in turn, leading to impaired lymphatic drainage. Again, neoplasia cannot be fully ruled without cytologic or histopathologic assessment. If surgery is pursued, consider submitting a lymph node for histopathology to better define the nature of the lymphadenopathy present.
– The prostatic changes are most consistent with benign prostatic hyperplasia, a common finding in older intact male dogs. This can be difficult to distinguish from prostatitis in some cases. Given the persistent fever, echogenic urinary debris and evidence of bacteriuria and pyuria, septic prostatitis cannot be fully excluded. Continue assessing urinalysis as clinically indicated and consider urine culture and sensitivity if not recently performed. Pending C&S, continued antibiotic therapy (i.e. enrofloxacin) is recommended.
– There is scant ascites noted in close proximity to the right adrenal gland. This is more likely inflammatory in nature, however, given adreno-caval invasion, acute hemorrhage is a risk. If surgery is not an option or is delayed, monitor for increased discomfort, development of pale mucous membranes, abdominal distension, etc. which could indicate hemorrhage.
– There is mild punctate mineralization noted throughout the renal cortices, adrenal glands, and lymph nodes. This is likely dystrophic in nature.
– The duodenal changes are mild. Continue GI supportive care as clinically indicated (i.e Cerenia, Ondasetron, Reglan, etc.).
– If unable to pursue further treatment, medical management could be considered, however, clinical improvement is unlikely. Consider pain medication for palliation in addition to gastrointestinal medications previously discussed. Rocko may also benefit from starting Clopidogrel to aid in prevention of a thromboembolic event.
– Consider other diagnostics/therapeutics as clinical signs dictate.
Outcome
Unfortunately, the owner elected for humane euthanasia due to quality of life, disease progression, and prognosis.
Discussion
The adrenal glands are endocrinologically active organs which contain two functional units: the cortex and medulla. The cortex produces steroid hormones (glucocorticoids, mineralocorticoids, and androgens) while the medulla primarily secretes catacholamines. Ultrasonographic changes to the adrenal glands are not uncommon in dogs. These changes in appearance range from atrophied and flattened adrenals to large masses, and everything in between (including a combination thereof). Similarly, the associated underlying pathology (and clinical significance) ranges from normal physiologic variation to highly malignant and metastatic neoplasia. While ultrasonographic findings provide clinicians with valuable insights into potential differentials and help inform subsequent diagnostic and therapeutic steps, a comprehensive approach—integrating imaging, clinical signs, lab results, and functional adrenal testing—is often essential to fully understand the significance of the observed changes.
There are multiple published studies attempting to identify differentiating characteristics of adrenal pathology using varying imaging modalities to better determine an underlying etiology. Multiple studies⁵⁻⁷, have identified adrenal mass size as a predictor of malignancy in dogs. In the few studies that attempt to establish adrenal sizes as a differentiating characteristic,5,7 ≥ 2 cm was proposed as a cut off as 100% of the patients which met this criteria and subsequently obtained a histologic diagnosis were confirmed to have malignant neoplasms. Using this size criterion alone will likely yield poor sensitivity, nevertheless, can help determine the appropriateness of more invasive diagnostic/therapeutic options (i.e. adrenalectomy) when masses exceed this cut off. Similarly, vascular invasion was found to be specific but not a sensitive predictor of malignancy.7 Based on ultrasonographic assessment alone, size and invasive behavior of adrenal masses appear to be the only reliable criteria which have been identified to correlate with benign or malignant lesions. It is worth noting that some studies have demonstrated good diagnostic accuracy in differentiating adrenal tumor types and malignant versus benign adrenal lesions with the use of contrast enhanced ultrasonography (CEUS). One study found that combining contrast enhancement degree with vascularity allowed for a predictive model that differentiated adenocortical adenoma, adenocarcinoma, and pheochromocytoma with an accuracy of 91.7% (P = 0.001). Mirroring that accuracy, CEUS was able to discriminate between malignant vs. benign lesions.8 CEUS is currently not widely utilized/available, however, may become more prevalent in the future.
In this case, we see an example of an adrenal mass which exceeded 2 cm with invasion into the vena cava. As just outlined, the size and biologic behavior of this lesion is most consistent with a malignant, neoplastic process. As discussed within the report, the most commonly described invasive adrenal tumors are functional cortisol secreting tumors (adenocarcinomas) and pheochromocytomas. Given contralateral adrenomegaly and lack of ultrasonographic changes commonly associated with hyperadrenocorticism, a pheochromocytoma would be more likely in this case. Further diagnostics would be necessary for a diagnosis. In cases where malignant neoplasia with vascular invasion is suspected, unfortunately, the diagnostic and therapeutic options are limited if an attempt at long term survival is pursued. In one retrospective study, looking at outcomes of dogs who elected medical management of adrenal masses with vascular invasion, ~94% died or were euthanized with a median survival time of 50 days.¹ Reported survival times for patients that undergo adrenalectomy are highly variable with the majority of mortality occurring perioperatively. One study reported perioperative mortality to be as low as 15.4% with a median survival time of 953 days.9 However, other studies report perioperative mortality to be upwards of 60%.10 Multiple other articles report perioperative mortality rates to be within the 20-30% range.11-15 A study published in JAVMA in 2013 reported fatality within the short term period was high, with fourteen-day survival rates of 79% and 48% for carcinomas and pheochromocytomas respectively.² Moreover, caval invasion appeared to be a significant risk factor influencing short term survival (Hazard ratio 2.29; P = <0.001).² When further analyzed and subdivided into local and extensive caval invasion groups, the degree of caval invasion was significant with a 43% short term fatality rate for locally invasive tumors and 100% fatality rate for dogs with extensively invasive tumors (Hazard ratio 4.43; P = <0.001).² In this paper the extensive invasion was defined as tumor thrombi extending cranial to the hepatic portion of the vena cava, which this patient would be classified into. Additional univariate analysis identified pheochromocytoma as a risk factor affecting short term survival (Hazard ratio 1.75; P = 0.008),² however, multivariate analysis assessing tumor type (adenocarcinoma vs. pheochromocytoma) with both caval invasion (caval invasion vs no caval invasion), as well extent of caval invasion (local vs extensive), no longer demonstrated tumor type as a significant risk (P = 0.409 and 0.192 respectfully). Further advanced imaging (CT scan) and consultation with a veterinary surgeon would be necessary to assess surgical feasibility, however, statistical outlook for long- and short-term survival appears poor to guarded at best.
In cases of non-invasive adrenal pathology and lesions < 20 mm, preliminary diagnostics are warranted to further clarify the significance of the pathology seen before pursuing more invasive procedures such as adrenalectomy. This allows for a more cost effective and less invasive approach while limiting the overall risk to the patient, in order to better determine the optimal diagnostic and treatment plan. Functional cortisol secreting tumors account for ~15% of cases of hyperadrenocorticism in dogs and ~50% of those cases are malignant tumors.⁴ While a functional adrenocortical neoplasm (adenocarcinoma vs. adenoma) would be less likely in this case, in many cases, functional adrenal testing (ACTH stimulation test, LDDST, HDDST, Urine Cortisol:Creatinine ratio, Endogenous ACTH) are viable and necessary steps to better clarify the significance of both unilateral and bilateral adrenolomegaly with varying degrees of diagnostic accuracy. See the Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal)16 for further guidance in the diagnosis of HAC.
Additional diagnostics are necessary to aid in the diagnosis of a pheochromocytoma when surgical removal and histopathology are not pursued (or is not considered warranted based on the criteria aforementioned). Based on statistical prevalence, investigation into adenocortical disease should be investigated as well when an adrenal mass is seen. Many times, further investigation into pheochromocytomas is pursued when an unexpected adrenal mass is noted, the patient lacks clinical signs of underlying HAC, functional adrenal testing is not consistent with HAC, and/or there is a clinical suspicion of a pheochromocytoma (hypertension, weakness, collapse, etc.) In cases of pheochromocytoma, excess production of catecholamines (mainly epinephrine and norepinephrine) are generally released in an episodic or random manner.17 The metabolites of these compounds are metanephrine and normetanephrine (metabolites of epinephrine and norepinephrine respectively) which are continuously released. Because of this, the metabolites are a more reliable measurement than the catecholamines themselves. For a definitive diagnosis of a pheochromocytoma, histology is still considered gold standard, however, metanephrine testing can be used to strengthen the diagnosis in cases where adrenalectomy is not pursued or not possible. A recent study published in JAVMA, reported significant elevations in normetanephrine-to-creatinine ratios when compared to dogs with other adrenal tumors, with a sensitivity of 78.9% and a specificity of 76.9%.19 However, this study also found that the current standard for the diagnostic cut off (>4 times the upper reference range) only identified 5 of the 19 patients with pheochromocytomas.19 Free normetanephrine within the plasma has been found to be significantly higher in dogs with pheochromocytoma than healthy dogs, dogs with non-adrenal disease, and (importantly) dogs with adrenocortical tumors (averaging 63.89 nmol/L, 2.5 nmol/L, 3.3 nmol/L, and 2.96 nmol/L respectively).18 When used to diagnose pheochromocytoma, that same study found that a plasma free metanephrine concentration of 4.18 nmol/L yielded a sensitivity of 62.5% and specificity of 97.3%, and a plasma free normetanephrine concentration of 5.52 nmol/L had a sensitivity of 100% and specificity of 97.6%.18 Special handling is necessary for both tests and urine testing appears more readily available. Blood pressure should also be evaluated, however, given the episodic nature of catecholamine release, lack of documentation of hypertension does not rule out the presence of a pheochromocytoma.
The diagnosis of adrenal disease can range from straightforward in some cases to a rather turbulent pathway in others, often requiring a combination of imaging, clinical signs, laboratory results, and functional adrenal testing to reach a definitive conclusion. This case is a sobering example of the sometimes aggressive nature of adrenal disease, highlighting the critical role of ultrasound as a diagnostic tool in providing valuable insights for a more accurate and timely diagnosis.
Reference
- Fontes, Gabrielle S., et al. “Outcome in Dogs with Invasive Adrenal Gland Tumors That Did Not Pursue Adrenalectomy.” AVMA, American Veterinary Medical Association, 1 July 2024, avmajournals.avma.org/view/journals/javma/262/7/javma.23.12.0689.xml.
- Barrera, Jessica S., et al. “Evaluation of Risk Factors for Outcome Associated with Adrenal Gland Tumors with or without Invasion of the Caudal Vena Cava and Treated via Adrenalectomy in Dogs: 86 Cases (1993–2009).” AVMA, American Veterinary Medical Association, 15 June 2013, avmajournals.avma.org/view/journals/javma/242/12/javma.242.12.1715.xml.
- Gostelow, R., Bridger, N. and Syme, H.M. (2013), Plasma-Free Metanephrine and Free Normetanephrine Measurement for the Diagnosis of Pheochromocytoma in Dogs. J Vet Intern Med, 27: 83-90. https://doi.org/10.1111/jvim.12009
- Behrend EN: Canine hyperadrenocorticism. In: Feldman EC, Nelson RW (eds): Canine and Feline Endocrinology, 4th ed. Elsevier, St. Louis 2015 pp. 377-451.
- Cook, Audrey K., et al. “Clinical Findings in Dogs with Incidental Adrenal Gland Lesions Determined by Ultrasonography: 151 Cases (2007–2010).” AVMA, American Veterinary Medical Association, 15 May 2014, avmajournals.avma.org/view/journals/javma/244/10/javma.244.10.1181.xml?utm_source=chatgpt.com.
- Besso, J.G., Penninck, D.G. and Gliatto, J.M. (1997), RETROSPECTIVE ULTRASONOGRAPHIC EVALUATION OF ADRENAL LESIONS IN 26 DOGS. Veterinary Radiology & Ultrasound, 38: 448-455. https://doi.org/10.1111/j.1740-8261.1997.tb00870.x
- Pagani E, Tursi M, Lorenzi C, Tarducci A, Bruno B, Borgogno Mondino EC, Zanatta R. Ultrasonographic features of adrenal gland lesions in dogs can aid in diagnosis. BMC Vet Res. 2016 Nov 28;12(1):267. doi: 10.1186/s12917-016-0895-1. PMID: 27894345; PMCID: PMC5126813.
- Bargellini P, Orlandi R, Dentini A, Paloni C, Rubini G, Fonti P, Diana A, Peterson ME, Boiti C. Use of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Adrenal Tumors in Dogs. J Am Anim Hosp Assoc. 2016 May-Jun;52(3):132-43. doi: 10.5326/JAAHA-MS-6363. Epub 2016 Mar 23. PMID: 27008325.
- Massari F, Nicoli S, Romanelli G, Buracco P, Zini E. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002-2008). J Am Vet Med Assoc. 2011 Jul 15;239(2):216-21. doi: 10.2460/javma.239.2.216. PMID: 21756177.
- Scavelli TD, Peterson ME, Matthiesen DT. Results of surgical treatment for hyperadrenocorticism caused by adrenocortical neoplasia in the dog: 25 cases (1980–1984). J Am Vet Med Assoc 1986; 189: 1360–1364.
- van Slujis FJ, Sjollema BE, Voorhout G, et al. Results of adrenalectomy in 36 dogs with hyperadrenocorticism caused by adreno-cortical tumor. Vet Q 1995; 17: 113–116.
- Barthez PY, Marks SL, Woo J, et al. Pheochromocytoma in dogs: 61 cases (1984–1995). J Vet Intern Med 1997; 11: 272–278.
- Anderson CR, Birchard SJ, Powers BE, et al. Surgical treatment of adrenocortical tumors: 21 cases (1990–1996). J Am Anim Hosp Assoc 2001; 37: 93–97.
- Kyles AE, Feldman EC, De Cock HE, et al. Surgical management of adrenal gland tumors with and without associated tumor thrombi in dogs: 40 cases (1994–2001). J Am Vet Med Assoc 2003; 223: 654–662.
- Schwartz P, Kovak JR, Koprowsky A, et al. Evaluation of prognostic factors in the surgical treatment of adrenal gland tumors in dogs: 41 cases (1999–2005). J Am Vet Med Assoc 2008; 232: 77–84.
- Behrend, E.N., Kooistra, H.S., Nelson, R., Reusch, C.E. and Scott-Moncrieff, J.C. (2013), Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal). J Vet Intern Med, 27: 1292-1304. https://doi.org/10.1111/jvim.12192
- Nelson, R. W., & Couto, C. G. (2013). Small animal internal medicine - E-book (5th ed.). Elsevier. Chapter 53: Disorders of the adrenal gland, Section: Pheochromocytomas.
- Gostelow, R., Bridger, N. and Syme, H.M. (2013), Plasma-Free Metanephrine and Free Normetanephrine Measurement for the Diagnosis of Pheochromocytoma in Dogs. J Vet Intern Med, 27: 83-90. https://doi.org/10.1111/jvim.12009
- Waldron C, Thamm DH, Watson-Skaggs M, Vinayak A. High specificity and sensitivity of spot urine normetanephrine-to-creatinine ratios in the diagnosis of canine pheochromocytoma. J Am Vet Med Assoc. 2024 Nov 13:1-9. doi: 10.2460/javma.24.06.0409. Epub ahead of print. PMID: 39536456.


